INTRODUCTION
Canine cognitive dysfunction is a clinical condition, which is primarily identified by behavioural changes in the aged patient. In human medicine, single mono-ingredient supplements have proven successful in aiding symptoms that are normally associated with Alzheimer's. It has been hypothesised that nutritional supplementation can also be used in the management of the canine condition.
The clinical trial
This trial was designed to investigate the therapeutic effects of a specific supplement when compared to placebo. It was a randomised, multi-centered, double-blinded, placebo controlled clinical trial. Inclusion and exclusion criteria are outlined in figures 1 and 2.
Trial design
The trial was conducted through 20 UK veterinary practices. 24 dogs were enrolled in the placebo group and 20 in the "Akitvait" group. Owners were asked to record the incidents and severity of the behaviours indicative of cognitive dysfunction over a 56-day period, using a daily questionnaire. A baseline score for each was established after a 7-day pre-trial assessment. This was followed by administration of "Akitvait" or placebo for 42 days. Finally there was a further 7-day post trial assessment without administration of capsules. During 56 days the owners attended five face-to-face and two phone consultations.
Daily scores
Owners were asked to record daily scores relating to specific behaviours indicative of canine cognitive dysfunction. The scores are detailed in figure three.
|
Figure 1. Inclusion Criteria
 Dogs over 8 years of age Dogs over 8 years of age
 Been in the owner's possession for at least two months Been in the owner's possession for at least two months
 Been displaying signs of cognitive decline for at least one month Been displaying signs of cognitive decline for at least one month
 Male or female Male or female
 Neutered or entire Neutered or entire
 Any breed Any breed
 The dog must display behavioural symptoms of cognitive dysfunction The dog must display behavioural symptoms of cognitive dysfunction
 Those signs must include those associated with disorientation Those signs must include those associated with disorientation
 In addition the dog must show signs from at least one of the following categories In addition the dog must show signs from at least one of the following categories
 Changes in social interaction Changes in social interaction
 Changes in sleep/wake cycle Changes in sleep/wake cycle
 Alterations in house soiling incidents Alterations in house soiling incidents
|
|
Figure 2. Exclusion Criteria
 Dogs displaying signs of clinical disease on veterinary examination Dogs displaying signs of clinical disease on veterinary examination
 Dogs showing abnormal results on routine haematology or biochemistry Dogs showing abnormal results on routine haematology or biochemistry
 Dogs receiving current treatment in relation to old age behaviour change, e.g., selegiline hydrochloride, nicergoline, propentofylline, nutritional supplements or specific prescription diets for control of the symptoms of old age Dogs receiving current treatment in relation to old age behaviour change, e.g., selegiline hydrochloride, nicergoline, propentofylline, nutritional supplements or specific prescription diets for control of the symptoms of old age
 Dogs exhibiting any behavioural problems relating to disorientation, social interaction, sleep patterns of house training prior to 8 years of age Dogs exhibiting any behavioural problems relating to disorientation, social interaction, sleep patterns of house training prior to 8 years of age
 Dogs showing an appreciable level of aggression toward people which has already resulted in physical injury however minor or has caused the owners reasonable concern that physical injury may occur. Dogs showing an appreciable level of aggression toward people which has already resulted in physical injury however minor or has caused the owners reasonable concern that physical injury may occur.
|
|
Figure 3. Daily Scores
 Number of incidents of lack of recognition of people, other animals or places per day Number of incidents of lack of recognition of people, other animals or places per day
 Number of incidents of altered social interaction from or toward the dog per day Number of incidents of altered social interaction from or toward the dog per day
 Number of nights per week that the dog displays restless or broken sleep patterns Number of nights per week that the dog displays restless or broken sleep patterns
 Number of incidents of inappropriate toileting behaviour per week Number of incidents of inappropriate toileting behaviour per week
 Number of locations used for inappropriate toileting Number of locations used for inappropriate toileting
 Number of substrates used for inappropriate toileting Number of substrates used for inappropriate toileting
|
Additional data collection
In addition to the daily scores, global scores of each criterion (disorientation, social interaction, sleep patterns and house soiling) were also recorded on days -7, 0, 10, 21, 28, 42 and 49. Overall assessment questions were also asked to both the owner and the veterinary surgeon or nurse relating to overall improvement of behavioural symptoms relative to day 0 of the trial, improvement in the pet-owner relationship and improvement in the pet's quality of life.
Results
Two cases in the Akitvait group and one in the placebo group failed to complete the trial leaving 23 placebo and 18 Akitvait cases to be used in the statistical analysis of results. The data used in the analyses were the differences compared to the pre-trial week for the daily scores and the first day of the trial for all other measures.
For the Global Assessment Scores, the data were coded on a numerical scale:
 Insignificant = 0
Insignificant = 0
 Mild = 1
Mild = 1
 Significant = 2
Significant = 2
 Moderately severe = 3
Moderately severe = 3
 Very severe = 4
Very severe = 4
For the questions to the owner and the vet/nurse, data were again coded into a numerical scale:
 Significant improvement = 3
Significant improvement = 3
 Moderate improvement = 2
Moderate improvement = 2
 Mild improvement = 1
Mild improvement = 1
 Unaffected = 0
Unaffected = 0
 Mild deterioration =-1
Mild deterioration =-1
 Severe deterioration =-2
Severe deterioration =-2
Analysis
The main analytical method used is repeated-measures ANOVA. Unless otherwise stated, variances were homogeneous (as tested by Box's M) and residuals were always approximately normally distributed. The daily scores for each category were analysed after the removal of severe outliers, as identified using standardised residuals and Cook's distance. These outliers were not always cases that improved the significance of the results and in general placebo cases showed more extreme improvements or relapses while Akitvait treated cases tended to be within the bounds of acceptable variation. Outliers were not identified in every category and no more than 2 cases (4.5%) were found to be outliers in any one analysis.
Daily score results
At the start of the trial all dogs were sleeping for at least 7 hours during the day. The analysis of the daytime sleep pattern scores (after removal of outliers) showed that dogs receiving Aktivait are likely to be active for an extra two hours per day by day 42 of the trial--a statistically significant improvement of 30% while those dogs receiving placebo showed very little alteration in their daytime sleeping pattern and slept for only 8 minutes less by the end of the trial (See figure four).
Analysis of the daily scores for recognition behaviour showed that the number of incidents of lack of recognition in dogs receiving Aktivait were decreased from two to one incident per day while those dogs in the placebo group which showed two incidents per day at the start of the trial showed a reduction of one incident every third day. Differences and the placebo group were statistically significant.
Global assessment results
Global Assessment Scores were analysed for improvements in disorientation, social interaction, sleep patterns and housetraining and in addition they were analysed in a combined form, with the component scores being added together.
The house training global assessment revealed that at the start of the trial the average level of house training problems in the Aktivait treated group was significant while the average level in the placebo group was mild. By the end of the trial the average level in both groups was mild which represented a significant decrease in the Aktivait group (see figure six).
| Figure 4. | 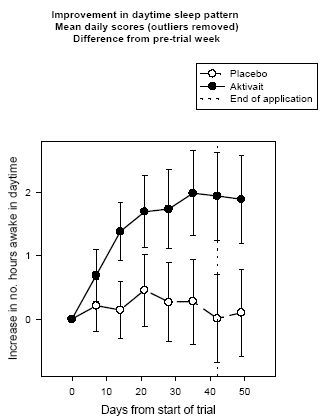
Improvement in daytime sleep pattern Mean daily scores (outliers removed) Difference from pre-trial week |
|
| |
| Figure 5. | 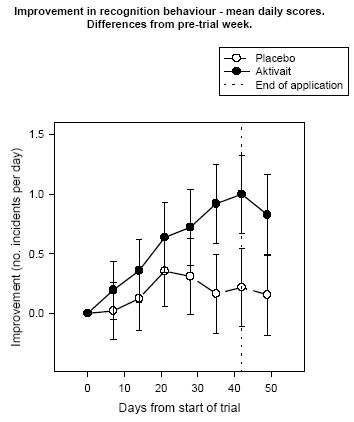
Improvement in recognition behaviour - mean daily scores. Differences from pre-trial week. |
|
| |
| Figure 6. | 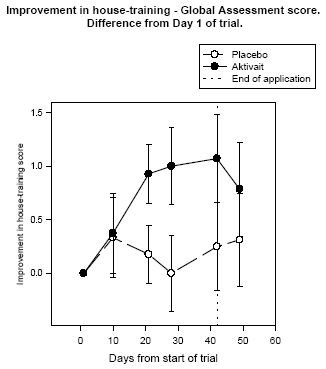
Improvement in house-training - Global Assessment score. Difference from Day 1 of trial.< |
|
| |
Assessments of overall improvement
Owners and veterinary surgeons were asked to record the overall improvement in age related behaviour and quality of life while owners were asked to make an additional assessment relating to their relationship with the dog. As well as being analysed individually, the scores were analysed in a combined form, with the component scores being added together to give an overall improvement as assessed by vets and by owners. The difference between the overall improvement in the Aktivait and placebo groups was statistically significant for both veterinary surgeon and owner assessment.
Conclusions
In this rigorous, multi-centered, double-blinded placebo controlled study "Akitvait" was proven to aid the management of dogs suffering from cognitive dysfunction behavioural symptoms. This is the first canine nutraceutical to be scientifically proven in this expanding field.