 Listen to this story.
Listen to this story.
Lesen Sie diesen Artikel auf Deutsch.
hormone dog
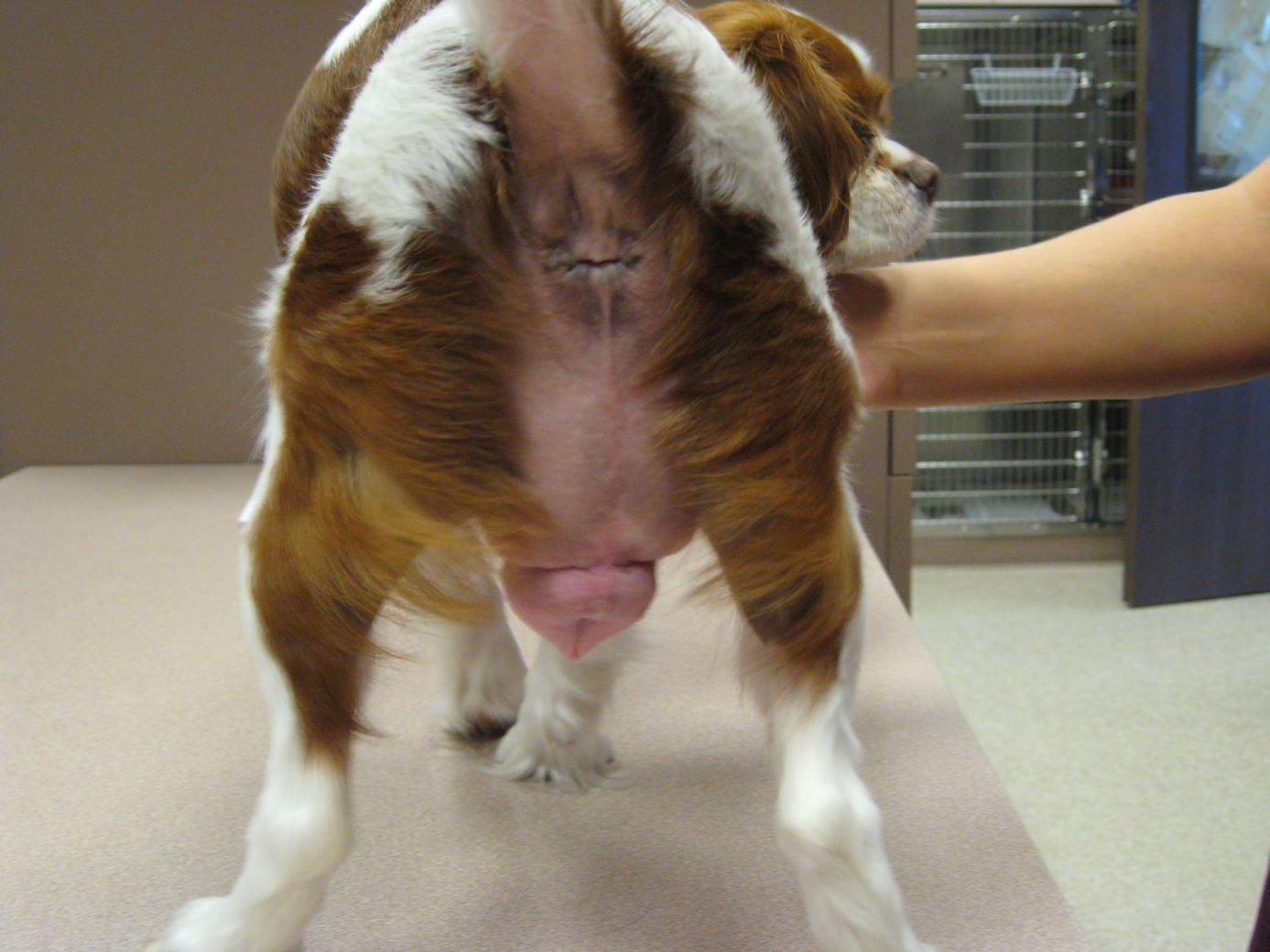
VIN News Service archive photo courtesy of Dr. Lisa Pope
More than a decade ago, a cavalier King Charles spaniel in Los Angeles experienced marked swelling of the genitals after being exposed accidentally to her owner's estrogen skin cream. The issue has gained attention more recently in Europe, as pets continue to be inadvertently exposed around the world.
The first report to capture Dr. Ann Neubert's attention involved a breeder of French bulldogs and Chihuahuas. Over the course of three months, four puppies were born with curious features. Three females had enlarged vulvas, while one male had swollen mammary glands. The apparent cause: exposure to a medication the breeder put on her skin containing the sex hormone estrogen.
As a veterinarian at the federal Office for Consumer Protection and Food Safety in Germany (Bundesamt für Verbraucherschutz und Lebensmittelsicherheit, or BVL), Neubert learned of the case through a report submitted in 2020 to the BVL by the Drug Commission of German Pharmacists (Arzneimittelkommission der Deutschen Apotheker). The commission records drug risks and evaluates complaints from pharmacies regarding drug quality and side effects.
Although she did not see the pups herself, Neubert quickly grasped the clinical implications: If these dogs had accidentally come in contact with their owner's transdermal hormone therapy and showed obvious effects, the same could be occurring with other household animals — and with children, as well.
"We can see our pets as a kind of indicator of what can happen with our children," Neubert told the VIN News Service in an interview. "Today, pets and children are treated kind of similar. So it's really important to have that [exposure risk] in mind."
To document the phenomenon and raise awareness among veterinarians in her country, Neubert and colleagues compiled findings in a study that was published last month in the journal Der Praktische Tierarzt.
The study adds to a growing, if still meager, body of research on an international phenomenon that reflects the popularity among human patients of hormone products applied to the skin in ointment, gel or spray form — usually estradiol (a form of estrogen) for menopausal and post-menopausal women and testosterone for men.
Though broadly unrecognized by the public, the issue is not new. VIN News began reporting in 2010 on accidental exposures to transdermal hormones. Over the following decade, VIN News tallied more than 150 cases in pets, mostly dogs. Most were anecdotal references made in posts on message boards of the Veterinary Information Network, an online community for the profession and parent of VIN News. Some cases were derived from correspondence and conversations with veterinarians and pet owners.
Awareness among veterinarians in the United States is higher now than a decade ago, judging from VIN message board discussions about the topic. But users of hormone products are less informed.
As recently as October, VIN News received an email from a person whose male dog's nipples became enlarged shortly after the person began hormone replacement therapy.
"I am so glad that I have an awesome vet who was aware of this issue," the dog's owner wrote.
Veterinarians are still learning the scope and consequences of exposures.
Larger dogs also susceptible; hairless cats, exquisitely so
Among dogs, small ones are considered more susceptible to the problem because they're more likely to be exposed to a larger dose relative to their size. But Neubert, the German government veterinarian, and her colleagues documented that larger dogs can be affected, too.
Among the reports the researchers reviewed were cases involving a vizsla, Weimaraner and Rhodesian ridgeback. The ridgeback was the heaviest, at 31 kilograms (68 pounds).
Small, short-haired dogs were represented more frequently, however, with the most common breeds being Chihuahua, Italian greyhound, pug, dachshund, basenji and Boston terrier.
Altogether, the German researchers reviewed the cases of 42 dogs. Most cases were derived from previously published studies, while 16 were from the EudraVigilance Veterinary database, a log of pharmaceutical adverse events reported in the European Union.
The most common clinical signs of exposure were swollen vulvas and hair loss (alopecia).
The paper also reported the cases of 13 cats, 11 of which were documented in the EU database.
Neubert said the feline data was revelatory in that clinical signs of exposure were found to be commonly behavioral and more severe than in dogs — sometimes leading to tragic ends.
One strikingly severe feline case was described in detail in a paper by researchers in Sweden published in the journal Veterinary Record in October 2022. Cases in that paper came from adverse event reports to the Swedish Medical Products Agency and the same EU pharmacovigilance database tapped by the researchers in Germany.
The unfortunate cat was one of three belonging to a woman who used a hormone spray on her upper arms twice daily for menopausal symptoms. According to the account, about two weeks after the owner began using the drug, the cat, a spayed, 3½-year-old sphynx — a hairless breed — began acting as though she was in heat, or estrus. She vocalized excessively, lost her appetite, raised her hindquarters and was "extra-affectionate."
A second cat in the household, a 2½-year-old castrated male sphynx, also began behaving differently. "Reported signs included anxiety, increased signs of territorialism and increased sexual interest in the female cat," the authors wrote.
Believing that the female neutered cat had retained ovarian remnants, her veterinarian prescribed the drug deslorelin, a synthetic sex hormone, to counteract the effects. But a month later, the researchers reported, she was worse: "[T]he signs progressed to include strong vocalisation, unusual territorial urination outside of the litter box, hyporexia [a prolonged decrease in appetite], insomnia and abnormal 'psychotic' behaviours."
The cat was then given the drug gabapentin, which acts on the nervous system. A month later, her "general health status ... had deteriorated." She was given the hormone progesterone. Her health worsened.
Exploratory surgery followed. The surgeon found no ovarian remnants. The owner ultimately elected to have the cat euthanized due to its poor health and unremitting exaggerated signs of estrus.
Later, the woman adopted a 12-week-old unneutered male sphynx kitten. Soon, she noticed that the kitten's mammary glands were enlarged. A veterinary exam found that the kitten also had unusually small testes.
It was at that point that the owner and veterinarian identified the owner's hormone therapy as a suspected cause of her cats' problems. The owner stopped using the therapy, the kitten was treated with the steroid aglepristone, and within a week, both male cats had improved and eventually recovered fully.
Another reported instance with fatal outcomes involved two exposed intact female cats. As recounted by the researchers in Sweden, the pair were unusually small, weighing between 2 and 3 kilograms (4½ and 6½ pounds) each. As Norwegian forest cats, a large breed, normal weight would have been 3½ to 7 kilograms (7.7 to more than 15 pounds), according to Neubert. Both cats gave birth to litters with stunted or stillborn kittens.
In a third instance described in the Swedish paper, an exposed intact female cat was similarly undersized. She gave birth to a kitten with myocele — a condition in which muscle protrudes through its sheath — malformed legs and paralysis. The kitten was euthanized.
(Neubert noted that the clinical signs in the mother cats and their offspring potentially might also be explained by other causes, including other types of environmental exposures, infections, genetic abnormalities and poor nutrition.)
Dr. Ann Neubert

Urbschat Berlin photo
A veterinarian in the federal Office for Consumer Protection and Food Safety in Germany, Dr. Ann Neubert is trying to educate clinicians and the public about the risk of accidental exposures in pets and children to topical medications containing estrogen or testosterone.
Spreading the word
In a drug safety bulletin prepared by Neubert and colleagues and posted by the Federal Institute for Drugs and Medical Devices in June 2022 to raise public awareness about accidental exposures to topical sex hormones, the researchers gave an overview of the issue, including statistics showing a distinct rise in the use of such drugs in human medicine.
Citing a national drug prescription report from 2021, the bulletin states that prescriptions for testosterone increased in Germany from 6 million defined daily doses in 2004 to 27.6 million defined daily doses in 2019. For transdermal estrogen, the defined daily doses increased 8.1% in 2020 compared with one year before, to 48.3 million.
The bulletin lists a number of cases reported in scientific literature or to the pharmacovigilance database of children, from infants to age 8, having been exposed to estrogen or testosterone products used by adults in their families. The children's symptoms included breast budding, premature puberty, sexual precocity, virilization, skin pigmentation and premature bone age.
Neubert told VIN News that unlike in the United States, where hormone therapies commonly are delivered as compounded preparations, the medications in Europe are commercial brands that have been vetted through a drug-approval process. Europe, she said, "is really strict compared with the U.S. It's a huge difference between our markets." Regardless, inadvertent exposures appear to have similar effects.
She noted that as of August 2022, estrogen sprays and gels prescribed throughout the EU must include instructions to users to avoid allowing children contact with the skin where the product has been applied. Product information also must list possible side effects from unintended exposure. However, the required caution does not mention pets.
Neubert believes instances of secondary exposure are likely much higher than the statistics reflect. "As with any pharmacovigilance data, a high rate of underreporting can be suspected and, therefore, known cases only represent the very top of the iceberg," she said.
Once clinicians are alert to the issue, addressing it is easy, Neubert and colleagues suggest in their paper. "A thorough anamnesis is an inexpensive and comparatively simple diagnostic method," they write, referring to taking a patient history. They add: "Other diagnostic methods such as vaginal or preputial cytology, skin biopsy, hormone assays and imaging may confirm the suspicion or be used to rule out differential diagnoses such as incomplete neutering or hormone-producing cysts or tumours."
Treatment is equally simple: "Therapy consists of avoiding exposure," the researchers write.
To spread the word about the phenomenon, Neubert said she's been speaking at veterinary conferences in Germany. Next month, she is scheduled to present a poster on her findings at a meeting in Berlin devoted to veterinary and human reproduction.
In addition, one aspect Neubert is interested in plumbing further is unintended exposure in pets to topical testosterone, for which she found no cases reported in the scientific literature. Journal reports she and colleagues have cited regarding testosterone involve secondary exposure to children only.