 Listen to this story.
Listen to this story.
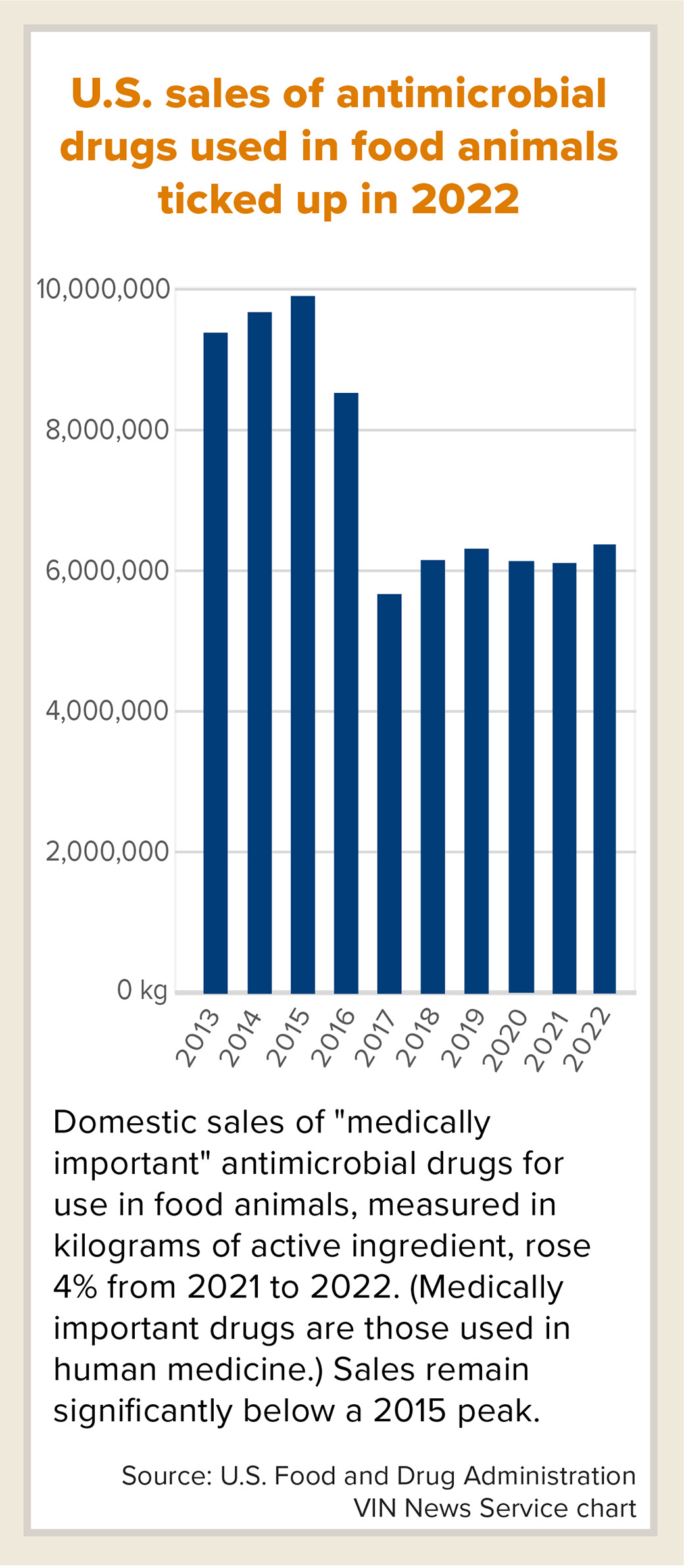
Sales of key antimicrobial drugs for use in food animals rose 4% in the United States in 2022 compared with 2021, contrasting with a steep decline reported in Europe and seemingly marking a setback in efforts to curb usage to combat pathogen resistance.
Still, the U.S. Food and Drug Administration noted that domestic sales were down 36% compared with 2015, when sales appeared to peak.
Purchases of "medically important" antimicrobials for use in production animals rose to a six-year high of 6,245,720 kilograms of active ingredient in calendar year 2022, up from a downwardly revised 5,989,721 in 2021, the FDA reported this month. Medically important antimicrobials are those that are also used in human medicine.
Health authorities and experts discourage indiscriminate use of antimicrobial medications — which include antibiotics, antivirals and antifungals — because it can contribute to the evolution of germs that withstand treatments meant to kill them.
Sales of medically important antimicrobials in the U.S. fell sharply in 2017 when federal regulators banned using the drugs to promote growth in livestock, prohibited over-the-counter use in animal feed and water, and required oversight by veterinarians.
Since then, sales have crept up. Still, the FDA indicated that this year's increase doesn't necessarily represent a trend. "Sales volume may fluctuate over time in response to various factors, including changing animal health needs or changes in animal populations," the agency said in a written statement.
FDA spokesperson Anne Norris said the agency can't speculate on why sales go up or down in a given year. Factors that can affect sales, such as disease prevalence and changes in animal populations, are outside the purview of its report, she said. "FDA stresses the importance of considering sales trends over time rather than focusing on one year in isolation," she cautioned.
Tetracyclines comprised the largest volume of U.S. sales, rising 4% year-on-year. The next most popular classes of antimicrobials, macrolides and aminoglycosides, rose 8% and 10%, respectively. Sales of penicillins fell by a fraction of 1%.
(The sales report focuses on products that have the potential to create cross-resistance concerns in humans, so it is not all-encompassing. Sales of antifungal, antiviral and antiprotozoal drugs without antibacterial properties are not included.)
Across the Atlantic, sales of veterinary antibiotic products in a combined 31 European countries dropped 12.7% in 2022 compared with 2021, according to the European Medicines Agency (EMA). For the 25 European countries that have provided sales data continuously between 2011 and 2022, aggregated sales dropped 53%. (Strictly defined, antibiotics are a subset of antimicrobial drugs, but the terms often are used interchangeably.)
The numbers in the U.S. and Europe are derived through different calculation methodologies.
The EMA reports sales of antimicrobial drugs in milligrams of active ingredient per "population correction unit," or PCU, a proxy that accounts for the size of the food-animal population. The amount sold in 2022 from 31 European countries was an all-time low of 73.9 mg per PCU, down from the 84.7 mg reported for 2021. (The countries encompass all 27 members of the European Union plus Norway, Switzerland, Iceland, Liechtenstein and the United Kingdom.)
Separately, the U.K. released domestic figures showing sales of veterinary antimicrobials "adjusted for animal population" fell 9% in 2022 compared with 2021, an all-time low, and were down 59% since 2014.
Dr. Kitty Healey, head of surveillance and antimicrobial resistance at the U.K.'s Veterinary Medicines Directorate (VMD), said the drop reflected years of effort by government, industry and the veterinary profession to reduce usage. "We have seen focused and collaborative action across the animal health and veterinary sectors become established and mature, in many cases, and this is reflected in the antimicrobial consumption trends in this report," she said.
All the same, Healey said antimicrobial resistance remains a challenge at national and international levels.
Efforts to measure use in companion animals gain momentum
Most efforts to combat antimicrobial resistance in the veterinary realm are focused on food animals, but practitioners increasingly are being pressed to cap their use of the drugs in companion animals, too.
Attention turns to minor species
The U.K. generally is more advanced in measuring antimicrobial use in companion animals than elsewhere. Annual sales figures released by its VMD, for instance, include dogs and cats. The VMD calculates a defined daily dose for those animals that is based on the average number of days they received an antibiotic throughout the year. By that measure, in 2022, sales of antibiotic products for dogs fell 15% compared with 2021. For cats, they fell 13%.
In the U.S., the FDA recently started funding research at the University of Minnesota College of Veterinary Medicine that is tracking antibiotic use in American pets. The researchers are collecting data through a series of national point-prevalence studies, which involve collecting standardized data from many locations at a given moment in time.
One finding, for example, was that on a single day in August 2021, of 2,599 dogs and cats seen at 52 hospitals around the U.S., 29% were prescribed at least one antimicrobial medication.
The University of Minnesota research team recently completed data collection for two new antibiotic use studies — one in dermatology referral practices and one in dental referral practices, they told the VIN News Service in an email. The data from those studies are being validated and analyzed now.
The researchers also are developing a Companion Animal Veterinary Surveillance Network (CAVSNET), which draws data on antimicrobial prescribing from electronic medical records at the school's teaching hospital and from 115 practices owned by Banfield Pet Hospital, a unit of Mars Inc. They said they anticipate bringing on an additional data provider in the new year but declined to identify them before a data-use agreement is in place.
Having input from more than one corporate practice group will enhance the quality of the results, the researchers said, while noting that CAVSNET collects more data than what is included in the point-prevalence studies, such as breed: "We can follow patients over time. We can use the data for surveillance of diseases and therapies (this is in the works)."
In the future, they added, the CAVSNET dataset could be used to answer a host of research questions, such as those pertaining to risk factors for common diseases or outcomes of various treatments.
Elsewhere, some large owners of veterinary hospitals voluntarily are beginning to measure their use of antimicrobials in companion animals, with a potential aim to set usage goals.
Mars, one of the biggest owners of veterinary practices worldwide, told VIN News last December that it was planning to submit peer review and publication in a scientific journal an "anonymized, high-level analysis" of its antimicrobial use throughout the company.
The paper has since been submitted to a journal, and replies made to a first round of comments, Dr. Ian Battersby, Mars' head of pharmaceutical stewardship, said last week in an email. The company is awaiting the results of a second review and hopes to have more to share about publication timing soon, Battersby said.
This story has been changed to remove the name of the journal to which Mars submitted its research paper. Mars requested the removal out of concern that identifying the journal would compromise the anonymous paper review process.